New Photopatterning Platform
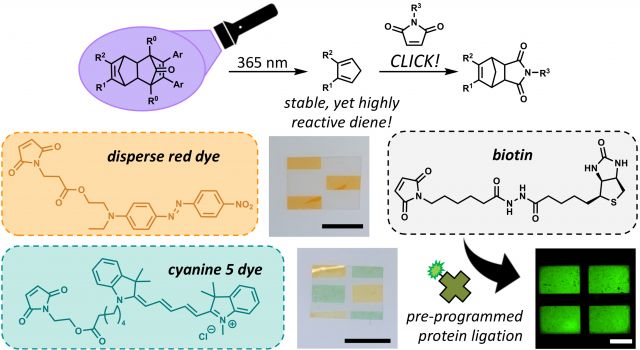
Adducts of cyclopentadienone–norbornadiene (CPD–NBD) have been developed and utilized in Diels–Alder based photopatterning of soft materials.
PI and Institution
Javier Read de Alaniz (UCSB)
Achievement
A new photopatterning platform which utilizes cyclopentadienone–norbornadiene (CPD–NBD) adducts as precursors to a highly reactive diene for Diels–Alder click cycloadditions has been developed. Upon UV light exposure, these CPD–NBD adducts undergo cleavage to cyclopentadiene, which will readily react with dienophiles to enable material patterning with a variety of species such as dyes (disperse red or cyanine 5) and biomolecules (biotin).
Importance of the Achievement
In comparison to previously established photopatterning platforms in soft materials, this platform provides a two-step approach in which the patterning agent (maleimide bearing species) can be introduced post-irradiation since the unveiled diene is a stable species. This is particularly useful as it expands the potential types of molecules which can be patterned. Particularly, photosensitive and biologically relevant molecules which are incompatible with current in situ photopatterning techniques may be patterned using this approach.
Unique Features of BioPACIFIC MIP that Enabled this Achievement
Financial support was provided for materials and supplies as well as for research personnel through the Fellows program.