Engineered Production of Strictosidine and Analogues in Yeast
Development of a yeast-based platform for high-titer production of the universal monoterpene indole alkaloids (MIA) precursor, strictosidine.
PIs and Institution
Joshua Misa, John M. Billingsley, Kanji Niwa, Rachel K. Yu, and Yi Tang (UCLA)
Achievement
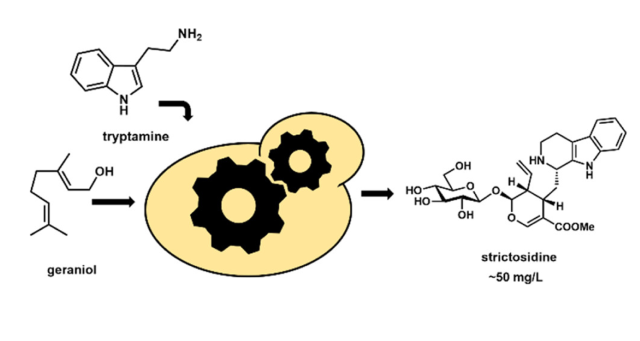
The use of microbial factories to produce high-value pharmaceuticals derived from plant natural products is enabled by recent advancements in synthetic biology and metabolic engineering. Yeast is an attractive host because it shares a similar endomembrane system with plants, which allows for heterologous expression of plant cytochrome P450 enzymes that are often responsible for generating the chemical complexity that confers potent biological activity. In this paper, the development of a yeast-based platform for high-titer production of the universal monoterpene indole alkaloids (MIA) precursor, strictosidine, is reported. The fedbatch platform is able to produce ∼50 mg/L of strictosidine, starting from the commodity chemicals geraniol and tryptamine. This was achieved by optimizing the expression of P450s and P450 accessory enzymes, decoupling the growth and production stages using auto-inducible promoters, and entering the pathway at high initial concentrations of commodity precursors. Moreover, the engineered host retains robust strain health, a key factor in strain development for lengthy MIA pathways.
Importance of the Achievement
MIAs are an expansive class of plant natural products, many of which have been named on the World Health Organization’s List of Essential Medicines. Low production from native plant hosts necessitates a more reliable source of these drugs to meet global demand. The strain improvements shown in this paper not only resulted in scalable titers that enabled the isolation of purified strictosidine, but also enabled the production of halogenated strictosidine analogues through the feeding of commodity chemical, tryptamine.
Unique Features of BioPACIFIC MIP that Enabled this Achievement
This work used the BioPACIFIC MIP Living Biofoundry facility for rapid preparation and incubation of yeast cultures which enabled high-throughput screening of promoter combinations. Moreover, BioPACIFIC MIP provided financial support for materials and researchers through the Fellows program.