Discrete Libraries of Amphiphilic Poly(ethylene glycol) Graft Copolymers: Synthesis, Assembly, and Bioactivity
Demonstration of a novel strategy for the scalable synthesis of multiple libraries of discrete lipid poly(ethylene glycol) (DMG-PEG) derivatives, with precise control over the structure and molecular weight.
PIs and Institution
Junfeng Chen, Aoon Rizvi, Joseph P. Patterson, and Craig J. Hawker (UCSB and UCI)
Achievement
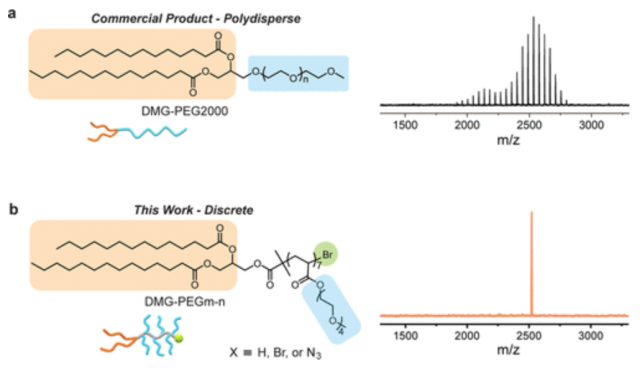
In this work, a novel strategy for the scalable synthesis of multiple libraries of discrete lipid poly(ethylene glycol) (DMG-PEG) derivatives, with precise control over the structure and molecular weight, is demonstrated. These DMG-PEG derivatives have a monodisperse backbone with side chains containing a discrete number of ethylene glycol units (3 or 4) and unique, functionalizable chain ends. Key to the success of this approach is the combination of controlled polymerization with automated chromatographic separation. A comparison with commercial linear DMG-PEG2000 conjugates, used in mRNA therapeutics, showed a similar ability to form lipid nanoparticles with nucleic acids and tunable/low levels of protein absorption. However, reduced anti-PEG antibody binding was observed compared to the commercial linear PEG materials, consistent with their branched and discrete structure. The availability of these discrete libraries enables the determination of fundamental structure/property relationships and comparisons with commercially relevant linear PEG derivatives.
Importance of the Achievement
PEG is an important and widely used polymer in biological and pharmaceutical applications for minimizing nonspecific binding while improving blood circulation for therapeutic/imaging agents. However, commercial PEG samples are polydisperse, which hampers detailed studies on chain length-dependent properties and potentially increases antibody responses. The development of discrete and sequence-specific synthetic macromolecules and control over their function and performance is a transformational opportunity in materials science, particularly in biological and pharmaceutical applications where single molecular entities are desirable.
Unique Features of BioPACIFIC MIP that Enabled this Achievement
This work made use of BioPACIFIC MIP’s Biotage Selekt automated flash purification system in the chromatographic separation of PEG derivatives, as well as the Matrix-assisted laser desorption/ionization (MALDI) system in the characterization of discrete oligomers.