Competition between β‑Sheet and Coacervate Domains Yields Diverse Morphologies in Mixtures of Oppositely Charged Homochiral Polypeptides
Aqueous mixtures of oppositely charged homochiral polypeptides reveal unexpectedly diverse morphologies at high polypeptide concentration and ionic strength.
PIs and Institution
Nairiti Sinha, Craig J. Hawker, and Matthew E. Helgeson, UC Santa Barbara
Achievement
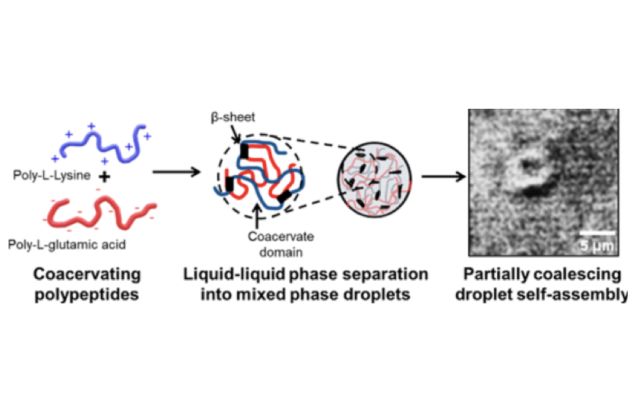
In this scientific paper, the authors investigated biomolecular assembly processes involving oppositely charged homochiral polypeptides in aqueous mixtures. Their investigation unveiled a spectrum of morphologies, including partially coalescing and aggregated droplets, bulk precipitates, and a previously unknown reentrant liquid-liquid phase separation at high polypeptide concentration and ionic strength. Through a combination of experimental results and molecular dynamics simulations, the researchers identified a concentration-dependent formation of β-sheet-rich secondary structures. This research revealed the delicate balance of interactions crucial for controlling coacervation morphology. Importantly, these insights not only contribute to a deeper understanding of similar biologically relevant contexts but also hold significant implications for technological applications involving biomaterials.
Importance of the Achievement
Highly charged peptides and proteins have been extensively investigated for their self and complex coacervation behavior, playing crucial roles in biological processes such as intracellular phase separation and in neurodegenerative disease pathways. From a technological perspective, researchers are exploring polypeptide coacervate materials for the discovery of superior biocompatible underwater adhesives and carriers for drug delivery. The study challenges existing coacervation theories, emphasizing the need to consider hydrogen bonding propensities, of peptide and protein backbones. These results elucidate a crucial balance of interactions that are important for controlling morphology during coacervation in these and potentially similar biologically relevant systems.
Synergies with BioPACIFIC MIP
This project made use of BioPACIFIC MIP specialized Zeiss microscope. This instrument proved vital for characterizing complex polypeptide morphologies, especially due to the challenging nature of samples involving combined turbidity and thickness.